Br Element Valence Electrons

Bromine has an atomic number of 35 and is part of the halogen group of elements and is considered a diatomic non metal.
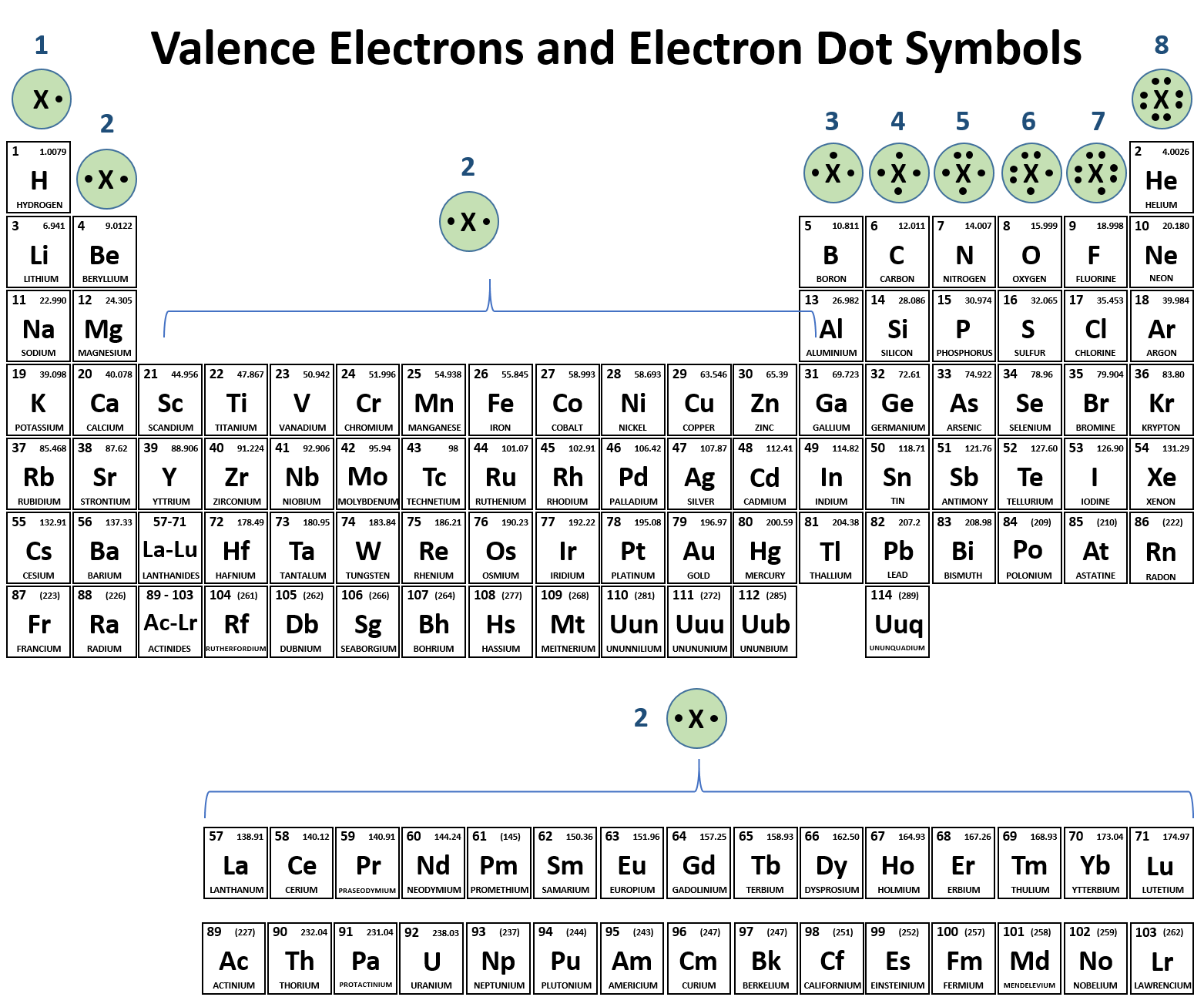
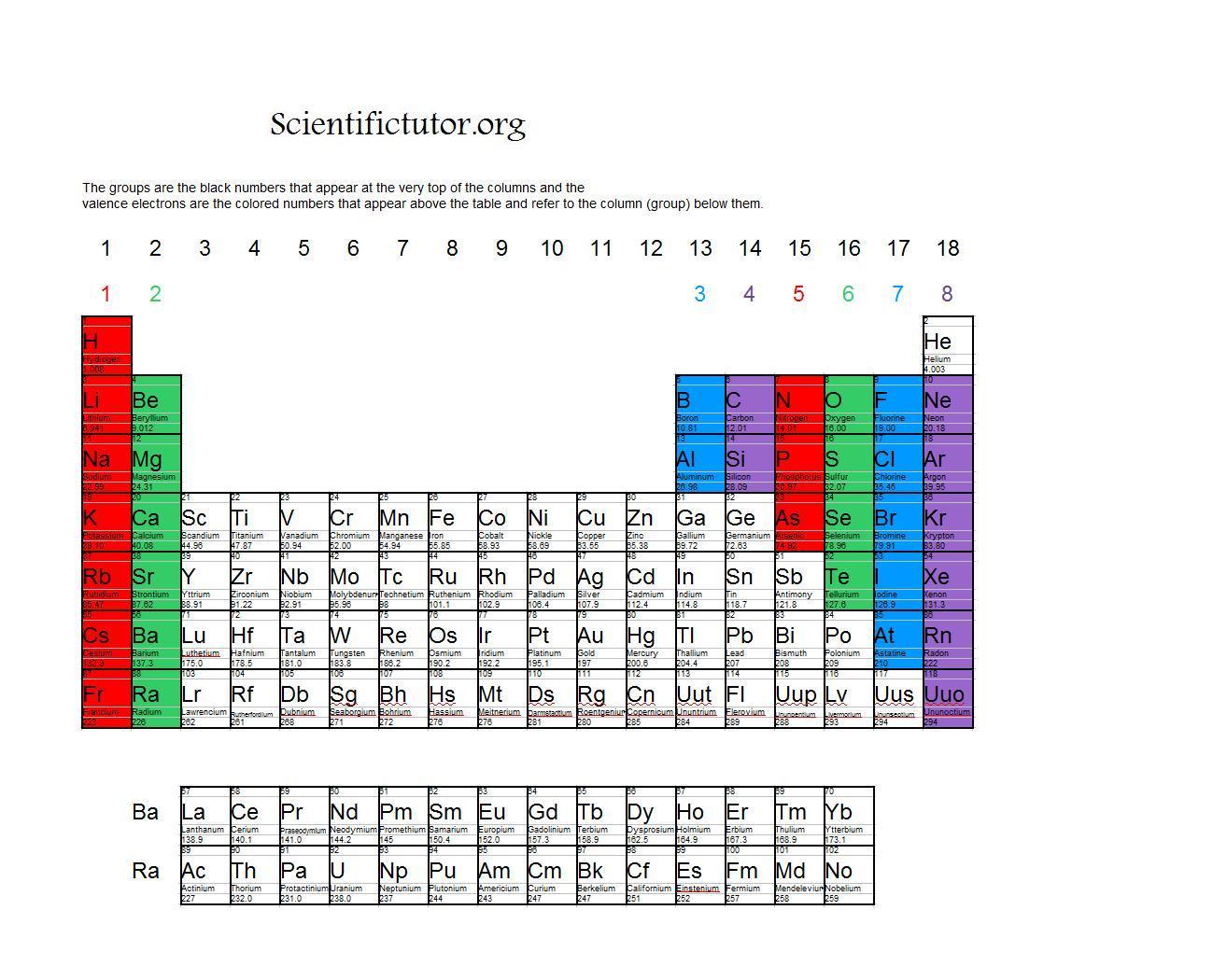
Br element valence electrons. Below is the lewis dot structure for a neutral bromine atom which has seven valence electrons. In chemistry valence electrons are the electrons that are located in the outermost electron shell of an element. Periodic table of elements with valence electrons trends. Valence electrons are the outermost electrons in an atom which affect how atoms might react with one.
In order for a bromine atom to become a 1 bromide ion it would have to gain an additional electron. Bromine is extracted by electrolysis from natural bromine rich brine deposits in the usa israel and china. Knowing how to find the number of valence electrons in a particular atom is an important skill for chemists because this information determines the kinds of chemical bonds that it can form and therefore the element s reactivity. The number reflects how many electrons an atom will accept negative number or donate positive number to form a chemical bond.
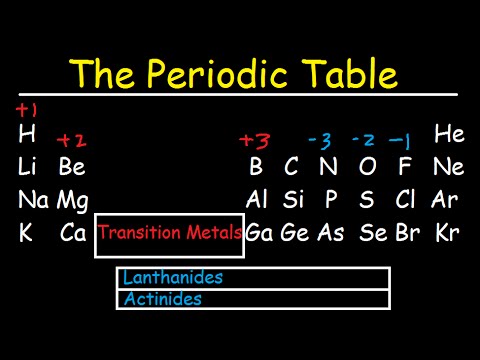
Valence is also known as oxidation state. It was the first element to be extracted from seawater but this is now only economically viable at the dead sea israel which is particularly rich in bromide up to 0 5. Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Like all halogens it is thus one electron short of a full octet and is hence a strong oxidising agent reacting with many elements in order to complete its outer shell.
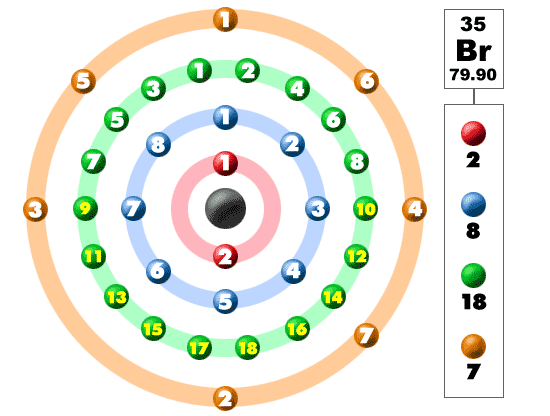
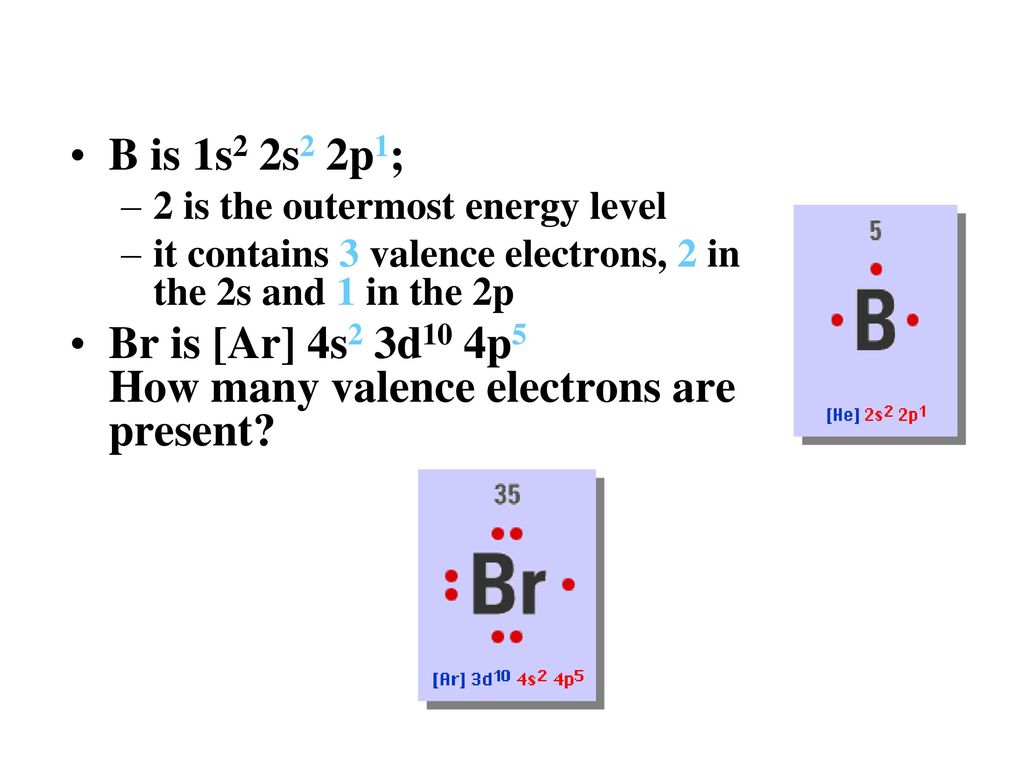
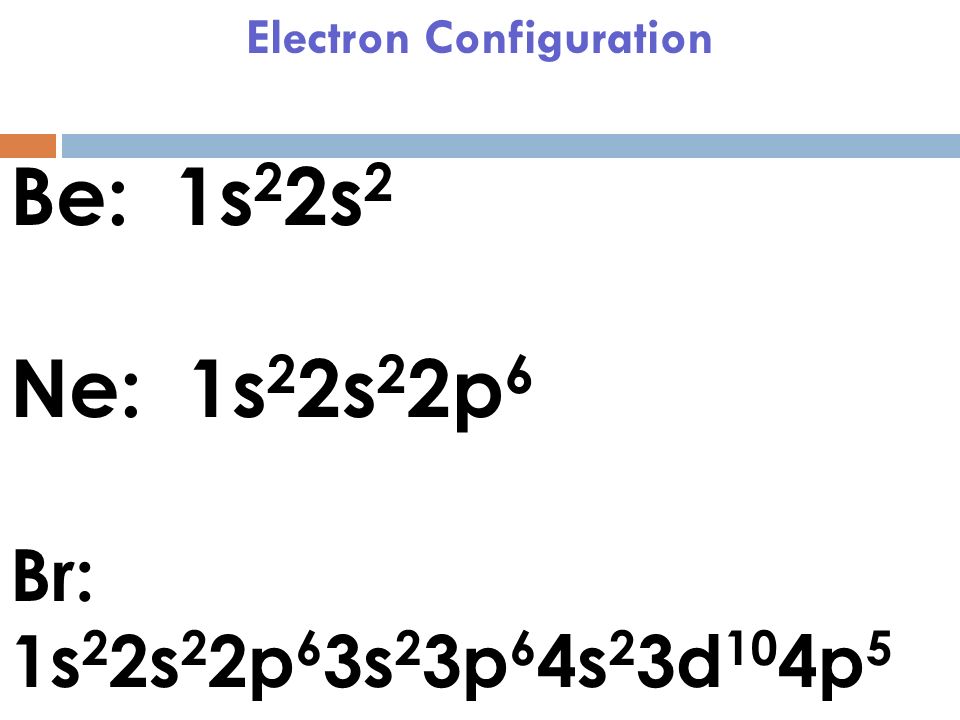
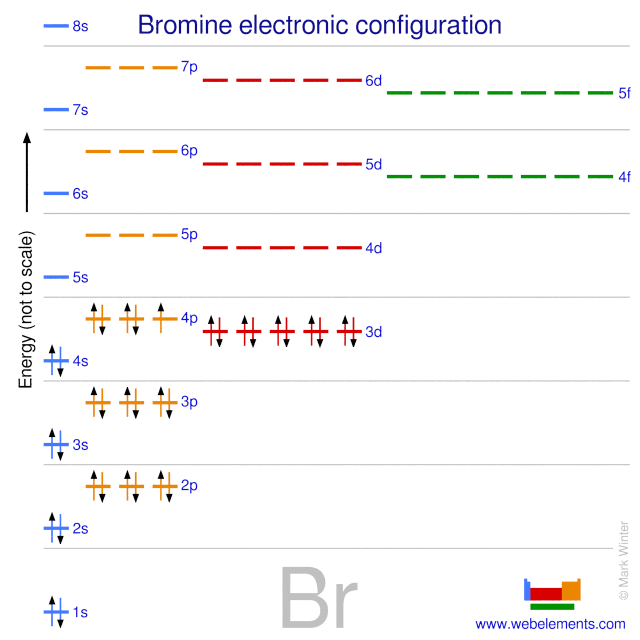
The atomic number for bromine is 35 which means it has 35 protons in its atomic nuclei. Bromine has the electron configuration ar 3d 10 4s 2 4p 5 with the seven electrons in the fourth and outermost shell acting as its valence electrons. This is a table of the valences of the elements. A neutral bromine atom would also have 35 electrons.
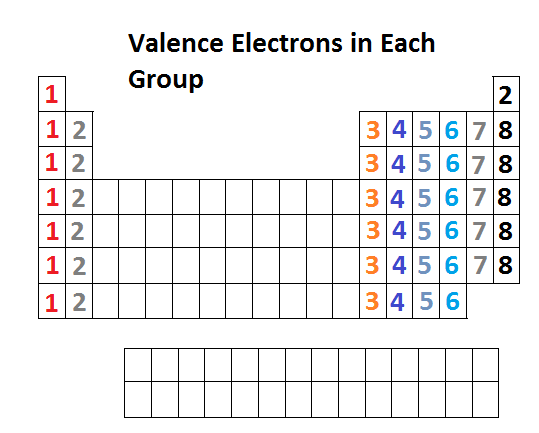
Whether it is electronegative or electropositive in nature or they indicate the bond order of a chemical compound the number of bonds that can be formed between two atoms. The br ion has 36 electrons. Natural salt deposits and brines are the main sources of bromine and its compounds. In the below periodic table you can see the trend of valence electrons.
It has seven valence electrons as indicated by the last part of the configuration 4s2p5. Jordan israel china and the united states are major producers of bromine. The most stable valence is one that fills or half fills an atom s electron shell. A bromine br atom has 7 valence electrons.
Bromine s chemical symbol is br.






.PNG)